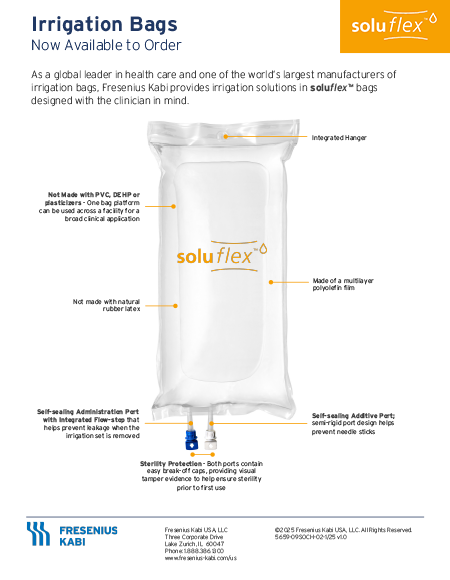
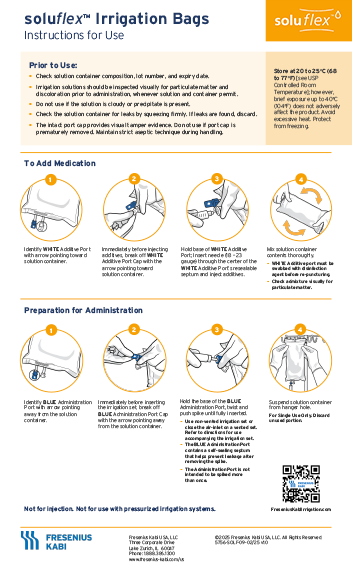
soluflex Irrigation Bags
Learn how to prepare and use soluflex irrigation bags
0.9% Sodium Chloride Irrigation, USP
0.9% Sodium Chloride Irrigation, USP is sterile, nonpyrogenic, isotonic and contains no bacteriostatic or antimicrobial agents.
0.9% Sodium Chloride Irrigation is indicated for all general irrigation, washing, rinsing and dilution purposes which permit use of a sterile, nonpyrogenic electrolyte solution.

Product Code
502010*
504020*
506030
NDC Code
65219-502-10
65219-504-20
65219-506-30
Fill Size (mL)
1000
2000
3000
Expiry
36-Month
36-Month
36-Month
Units per Case
6
5
4
Sterile Water for Irrigation, USP
Sterile Water for Irrigation, USP is a sterile, hypotonic, nonpyrogenic irrigating fluid or pharmaceutic aid (solvent) entirely composed of Sterile Water for Injection, USP. It is prepared by distillation and contains no antimicrobial or bacteriostatic agents or added buffers. The pH is 5.7 (5.0-7.0).
Sterile Water for Irrigation is indicated for use as an irrigating fluid or pharmaceutic aid. Sterile Water may also be used as an adjunct in the preparation of nonintravenously administered nutrient mixtures.

Product Code
495010*
497020*
499030
NDC Code
65219-495-10
65219-497-20
65219-499-30
Fill Size (mL)
1000
2000
3000
Expiry
36-Month
36-Month
36-Month
Units per Case
6
5
4
* Coming Soon
To order, go to MyFreseniusKabi.com
Important Safety Information
0.9% Sodium Chloride Irrigation, USP
CONTRAINDICATIONS: 0.9% Sodium Chloride Irrigation, USP is not for injection by usual parenteral routes. An electrolyte solution should not be used for irrigation during electrosurgical procedures.
WARNINGS: 0.9% Sodium Chloride Irrigation, USP is FOR IRRIGATION ONLY. NOT FOR INJECTION. Irrigating fluids have been demonstrated to enter the systemic circulation in relatively large volumes; thus, irrigation solutions must be regarded as systemic drugs. Absorption of large amounts can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.